So thats a subset so when Im talking about cis trans or geometric Im. A prefix used in the names of chemical compounds that are geometric isomers having two identical atoms or groups attached on opposite sides of a molecule divided by a given plane of symmetry.

Cis And Trans Isomerism Given Three Different Groups Around The Reference Plane Chemistry Stack Exchange
Difference Between Cis And Trans Isomers Definition Properties In Relation To The Structure
Cis Trans Isomerism Msrblog
So cis and trans isomers are diastereomers.
Cis and trans isomers. THEORITICAL BACKGROUND Cis-trans isomerism also known as geometric isomerism or configurational isomerism is a term used in organic chemistry. Cis-trans isomers belong to diastereomers since they are not mirror reflections of each other. Isomers are chemical compounds that have the same parts but are not the same.
For some you will be able to determine the relationship just by visual assessment some will require determining the R and S configuration there are also the ones where you compare different representations such as Newman vs Fischer or a bond-line vs sawhorse. The U shape of the cis isomer doesnt pack as well as the straighter shape of the trans isomer. The trans isomer cation has idealized D 2h point group symmetry whereas the cis isomer cation has C 2 symmetry.
Then the two identical methyl groups are either cis or trans to each other and the two identical hydrogen atoms are either cis or trans to each other. Isomerism the existence of molecules that have the same numbers of the same kinds of atoms and hence the same formula but differ in chemical and physical properties. This type of isomerism can arise in both organic and inorganic molecules.
Consider a simple case of geometric isomerism which weve already discussed on the previous page. Contrast this with stereoisomerism where isomers have the same atoms in the same order and with the same bonds but oriented differently in three-dimensional space. Recall cis and transThe reason Aldrich Chemical Co.
Recall That Cis-And Trans Isomers Geometric Isomers Cannot Interconvert Without Breaking Bonds. In the field of organic chemistry cis isomers contain functional groups on the same side of the carbon chain whereas the functional groups are on opposite sides in trans isomers. Trans- isomers generally have lower melting points and have lower densities than their cis- counterparts.
The key difference between cis and trans fatty acids is that the cis fatty acids have two hydrogen atoms attached to the double bond in the same side of the carbon chain whereas the trans fatty acids have the two hydrogen atoms bonded to the double bond in the opposite sides of the carbon chain. E-Z isomerism also known as cis-trans isomerism or Geometric isomerism is a type of stereoisomerism in which the same groups are arranged differently. This risks a tsk-tsk with accompanying finger-wag from IUPAC but it nevertheless gets the right structure.
However it is easy to find examples where the cis-trans system is not easily applied. Cis is when you have the two groups on the same side cis and trans is when you have the two groups on the opposite sides of the double bond. The general approach of the E-Z system is to observe the two groups at the end of each double bond.
Both trans-14-dimethylcyclohexane and cis-13-dimethylcyclohexane have essentially the same energy since neither one of them has any strain at all. This comparison brings an interesting question of whether we can ever have cistrans isomerism for a system with single bonds. The traditional system for naming the geometric isomers of an alkene in which the same groups are arranged differently is to name them as cis or trans.
This salt is more soluble than the cis isomer. Cis-trans isomers Question. Cistrans isomerism also known as geometric isomerism or configurational isomerism is a term used in organic chemistryThe prefixes cis and trans are from Latin.
Comparison of cis and trans isomers. Therefore cis and trans isomers are stereoisomers. At an introductory level in organic chemistry examples usually just involve the carbon-carbon double bond - and thats what this page will concentrate on.
These isomers occur where you have restricted rotation somewhere in a molecule. Cis-trans isomers exhibit a type of stereoisomerism where the atoms have different spatial arrangements in three-dimensional space. Learn more about isomerism in this article.
R-5-bromo-cis-2-hexene Please explain whether or not the following organic solvents could be used for. Geometric cis trans isomerism. This pair of isomers was significant in the development of the area of coordination chemistry.
When we talk about the difference between cis and trans isomers one of the main differences is in. Cis- isomers collect the charge on one side of the molecule giving the molecule an overall polar effect. In the following practice problems I put questions with different difficulty levels.
Structural isomerism is also known as constitutional isomerism. This side of and the other side of respectivelyIn the context of chemistry cis indicates that the functional groups substituents are on the same side of some plane while trans conveys that they are on opposing sides. Cis- isomers tend to have higher boiling points than their trans- counterparts.
You can tell which is the cis and which the trans form just by looking at them. Fatty acids are carboxylic acids containing long aliphatic carbon chains that are either saturated. However you cant because these are just two ways to represent the same molecule.
The ambiguity comes from the definition of similar groups. Rotation is energetically disfavored since it would destroy the overlap of the adjacent p-orbitals. The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon as in but-2-ene.
Cis and trans isomers are found both among organic and inorganic compounds. Trans- isomers balance the individual dipoles and have. In the context of chemistry cis indicates that the functional groups are on the same side of the carbon chain while trans conveys that functional groups are on opposing sides of the carbon chain.
The trans-13-dimethylcyclohexane isomer on the other hand has one methyl axial in both ring-flip conformers so that it is less stable than the cis isomer by 18 kcalmol. So this double bond has a cis configuration. Isomers and these are often called geometric isomers.
Difference Between Cis and Trans Cis-trans isomerism consists in the possibility of placing substituent groups on one or on different sides of a double bond plane or a non-aromatic cycle. Latin prefixal use of trāns preposition across through. The problem with the cis-trans system for naming geometric isomers.
Structural isomers are isomers that have the same component atoms but they are arranged differently from each other. Cis and trans isomers have the same molecular formula and molecular weight but they are known to differ in some aspects. Difference Between Cis and Trans Isomers.
Trans- synonyms trans- pronunciation trans. Lets compare the drawing on the left to the drawing on the right. And the answer is yes there are cis and trans isomers for systems with sigma bonds.
The first time you look at these two drawings you might think these are two isomers and I could use cistrans terminology to distinguish between them. The poorer packing in the cis isomers means that the intermolecular forces arent as effective as they should be and so less energy is needed to melt the molecule -. We can also use the cistrans nomenclature to distinguish isomers such as 2-methyl-3-hexene above rightIn the cis isomer the two hydrogens are on the same side of the pi bond and in the trans isomer the two hydrogens are on the opposite side of the bond.
Trans isomers pack better than cis isomers. Can sell 99 cis-2-butene and 99 trans-2-butene in separate bottles is because of restricted rotation about the C-C pi bond.

How To Draw And Name Cis Trans Isomers Yeah Chemistry
/25_DKPs_Cis_and_Trans_isomers.svg-5a4424f6494ec9003699ffd8.png)
Trans Isomer Definition

Cis Trans Isomers Definition Detailed Explanation With Examples
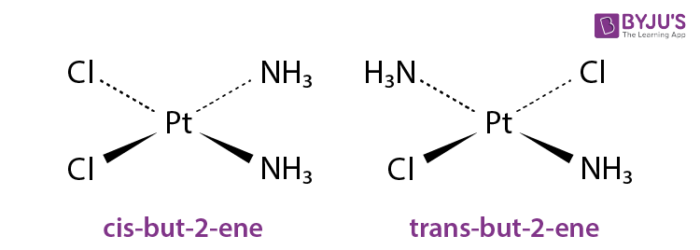
Cis Trans Isomers Definition Detailed Explanation With Examples

Organic Chemistry Cis Trans Geometric Isomerism A Level H2 Chemistry Tuition By 10 Year Series Author

Cis Trans And E Z Geometric Isomers Difference Explanation And Practice

Organic And Inorganic Chemistry Lesson Of The Day Cis Trans Isomers The Chemical Statistician
20 3 Naming And Drawing Cis Trans Isomers Kerem S Chemistry Notes Ib

